Take a Look at the Quality Assurance in QAPI
Survey results building up to the Phase 2 deadline of the ‘Mega Rule’ show how surveyors are grading skilled nursing centers on F-tag 520.
William M. Vaughan, RN
2/1/2018
Ask most people what the Affordable Care Act has to do with skilled nursing care centers, and the answers are likely to be centered on health insurance. Many would be surprised to learn that Section 6102 (c) of the Affordable Care Act directed the Centers for Medicare & Medicaid Services (CMS) to establish and implement a Quality Assurance Performance Improvement (QAPI) program for nursing centers. Phase 2 of the Requirements of Participation, commonly known as the “Mega Rule,” which went into effect Nov. 28, 2017, brought with it new regulations developed to meet this mandate.
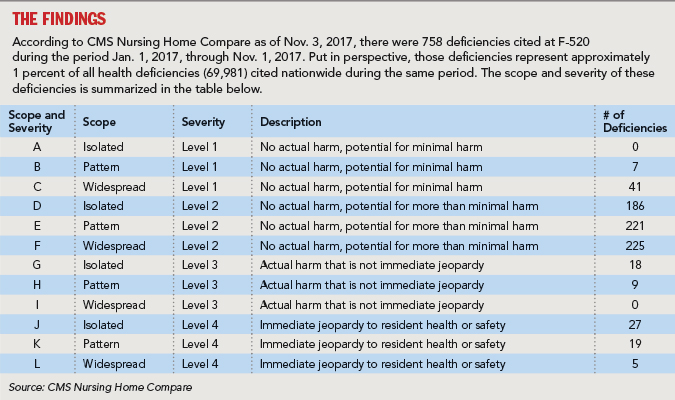
While the QAPI regulations significantly expand upon current quality assurance requirements, regulatory insight can be gained by reviewing what has been cited recently under F-tag 520 (Quality Assessment and Assurance).
It is notable that 27 deficiencies were cited at a severity level of “actual harm” (G, H) meaning that the surveyor determined the failure of the facility’s quality assurance process at least contributed to “harming” a resident. A review of the language from those deficiencies, cited in nursing centers from multiple states, revealed the following trends and patterns:
- The Quality Assurance (QA) Committee failed to develop and implement corrective action for pressure ulcer care and
- The facility’s failure to implement fall interventions resulted in Resident #30 sustaining a right femur fracture after a fall that caused actual harm.
- The facility failed to ensure the QA program was effective in preventing repeat deficiencies at F-323 (Facility Free of Accident Hazards) and F-514 (Clinical Records meet Professional Standards). The facility failed to prevent accidents, which resulted in a fall with a fracture for Resident #1.
- Based on record review and staff interviews, the facility failed to provide a comprehensive wound assessment, initiate treatment, and monitor a newly identified Suspected Deep Tissue Injury (DTI) to the left heel, resulting in worsening of the DTI. The recitation of F-314 (Treatment to Prevent/Heal Pressure Sores) and F-157 (Notify of Accidents/Significant Changes) during the past year of federal survey history showed a pattern of the facility’s inability to sustain an effective Quality Assessment and Assurance Review program.
- The facility failed to re-evaluate prior plans of correction to correct and maintain correction for previously cited deficient practice related to: environmental issues of fixing marred doors and walls; changing the tile in the resident rooms once marred, cracked, or missing; covering the bottom of the light bulbs for safety of the residents; notification of the dietician of a weight loss; the lack of documentation of supplements t
- The facility failed to identify and implement preventative measures to reduce the amount of facility-acquired pressure sores through a quarterly QAPI program for 1 of 4 quarters reviewed.
- The facility failed to ensure the QA Committee had an effective plan to monitor for continued compliance of deficiencies and issues identified and cited on previous recertification survey and complaint surveys.
Deficient practice at F-309 (necessary care for highest practicable well-being) was found again for failure to coordinate care regarding pain management and medication administration as documented for Resident #79 and lack of care coordination for Resident #257 regarding lack of communication between the Licensed Practical Nurse (LPN) and Registered Nurse (RN) concerning dehydration.
Deficient practice at F-514 (Clinical Records Meet Professional Standards) was found again cited regarding the facility’s failure to ensure clinical records were complete and accurate for 2 of 6 sampled residents by not accurately documenting a resident’s resuscitation status for 1 (Resident #180) of 1 resident reviewed for Hospice and not accurately transcribing a medication order for 1 (Resident #79) of 5 residents reviewed for unnecessary medications.
What This Means
Several themes emerge from this focused review of QA-related deficiencies. Not surprisingly, clinical processes and outcomes related to pressure ulcer development, falls with fractures, dehydration, weight loss, pain management, unnecessary medications, and resident rights continue to be regulatory barometers of quality.
Additionally, facilities that fail to follow plans of correction for previously cited deficiencies or are unable to maintain ongoing compliance with regulations face additional deficiencies that often prompt sanctions, including civil money penalties. While QAPI does represent a paradigm shift for both facilities and regulators, quality issues such as those described above tend to stand the test of time.

Lastly, facilities that actively involve their residents and surrogate decision makers in addressing these and many other quality issues will find that their processes are strengthened and regulatory compliance is more easily achieved.
William M. Vaughan was a surveyor with the Maryland State Survey Agency from 1988 until 2001. He became chief nurse of the agency in 2001 and remained in that position until joining Remedi SeniorCare in 2013. He is currently vice president of education and clinical affairs. He can be reached at 443-742-7173 or William.Vaughan@RemediRx.com.